Automation for the 21st century
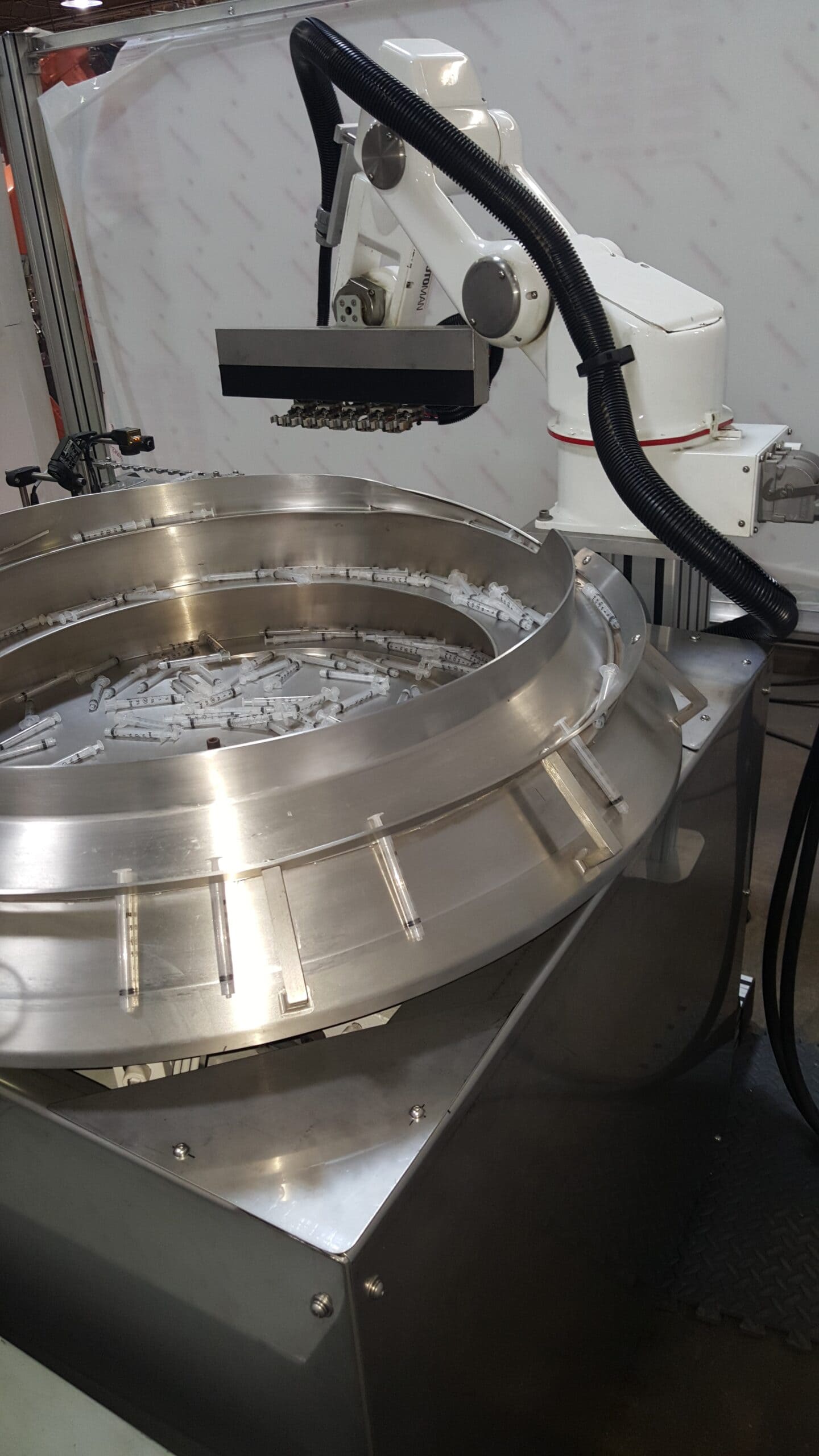
Rapid Development Services, is a leading provider of automated systems for medical device products. As a supplier of assembly, machine tending, and packaging machinery, RDS is quickly becoming a primary choice of medical manufacturers who need to automate their processes seeking exceptional customer service, historically reliable equipment, “one source solution” and attention to all the special details, required in “medical” product manufacturing.
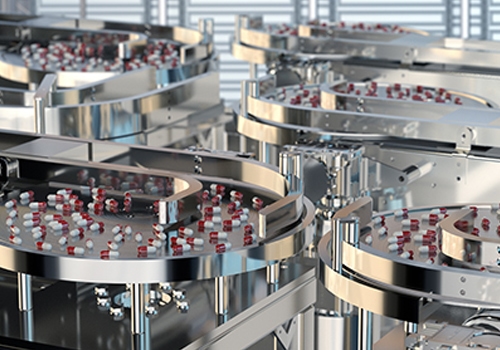
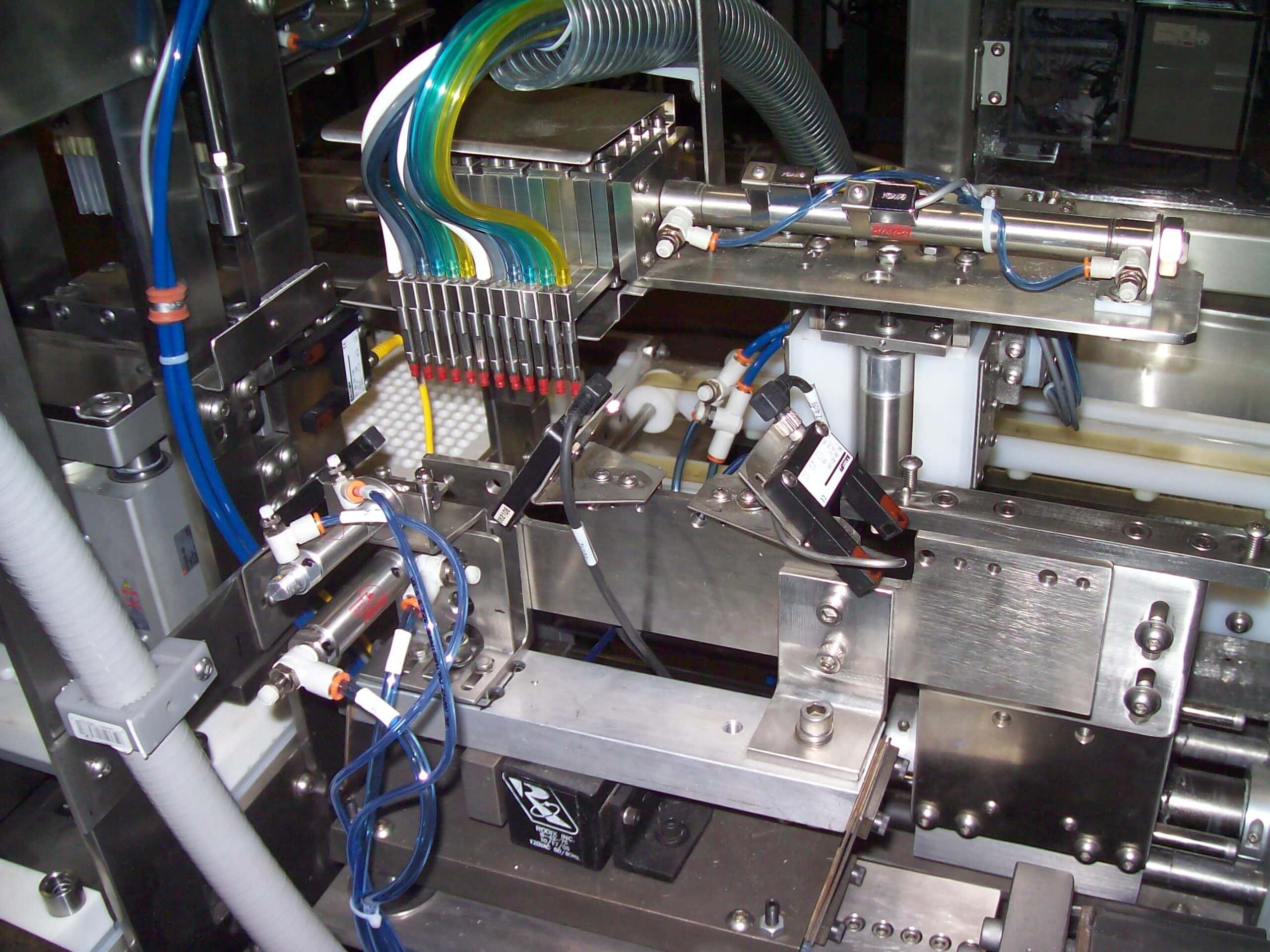
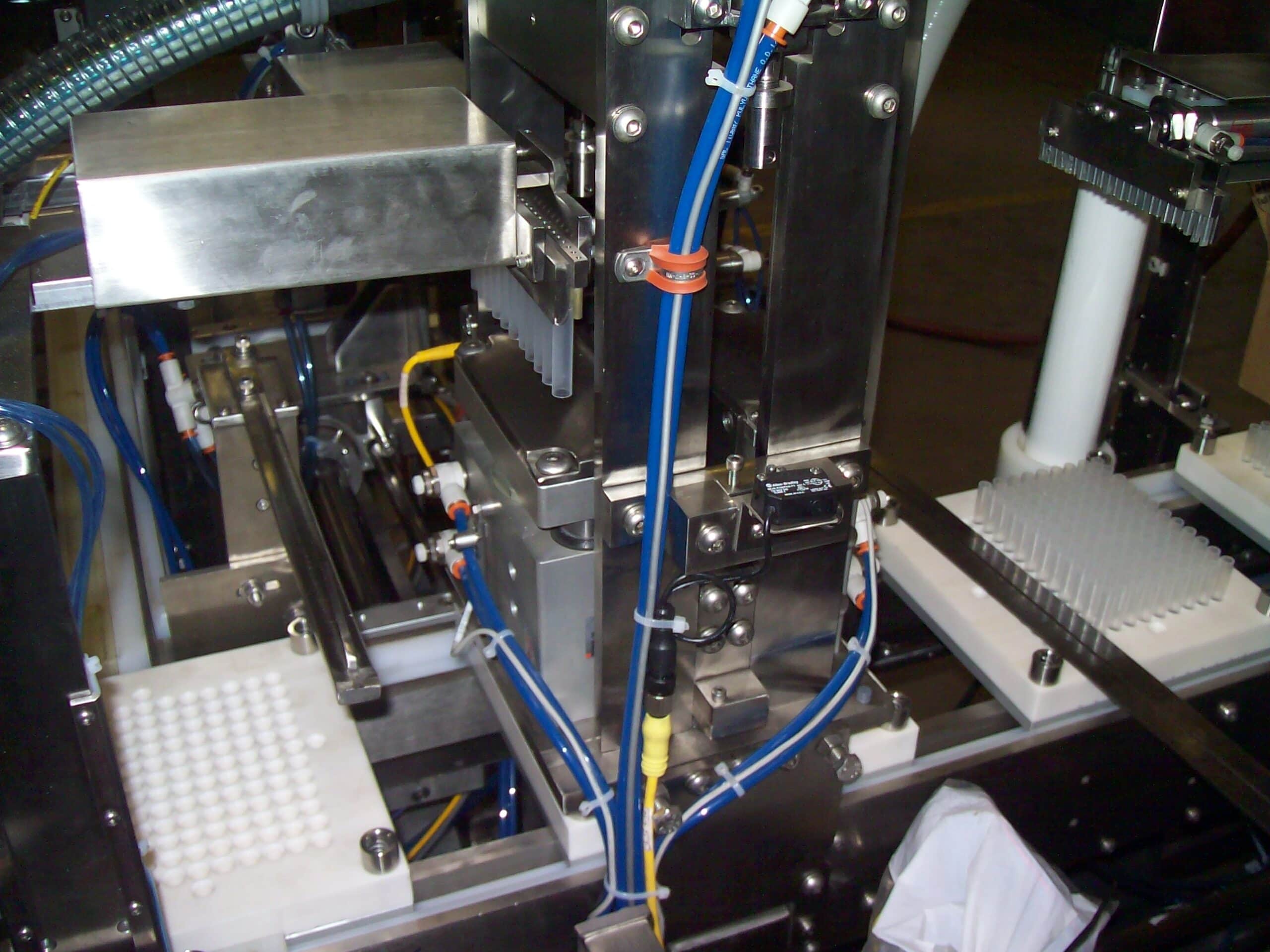
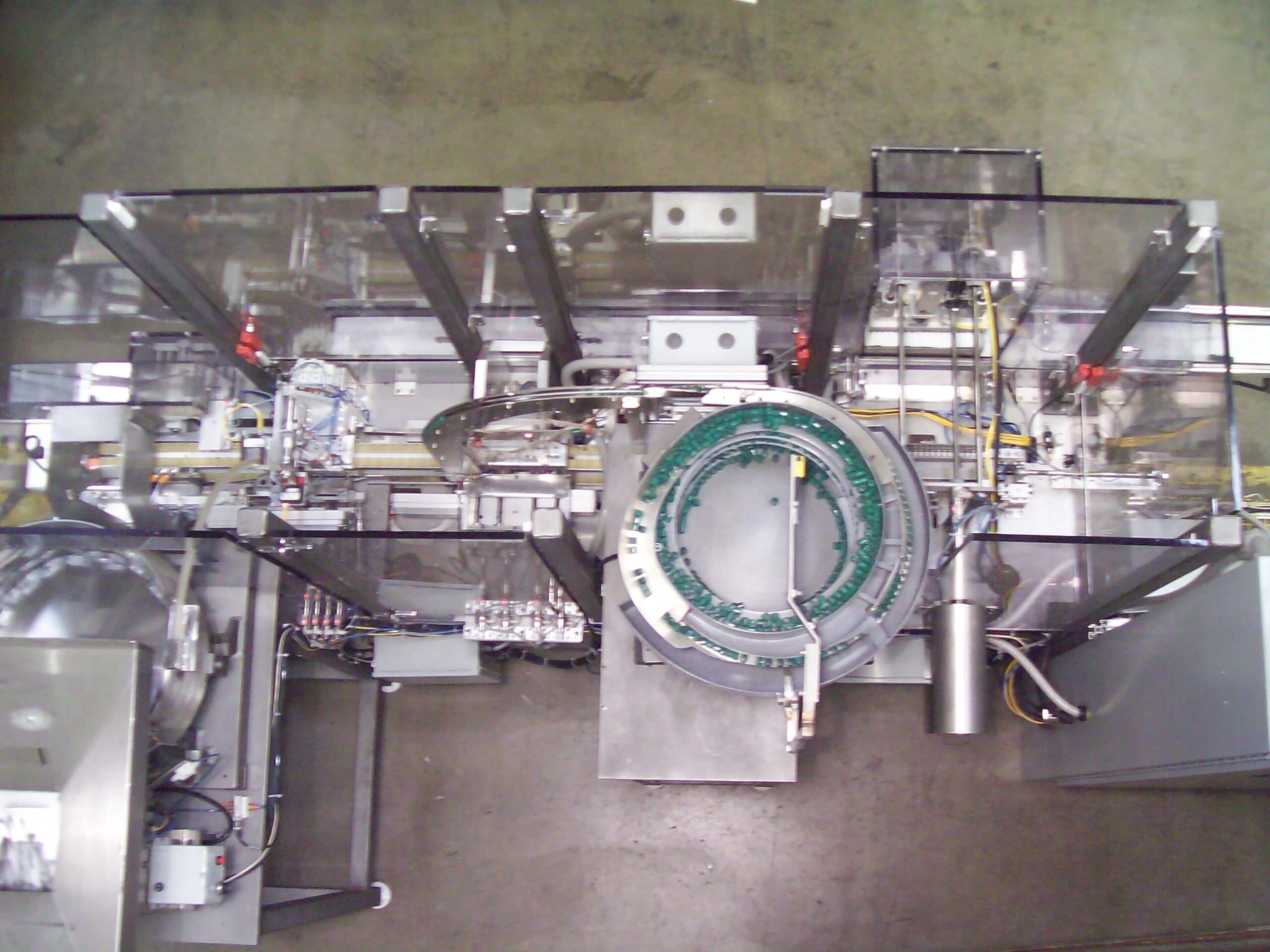
Advancement in medical technology has led to automation of medical device manufacturing and medical packaging applications. RDS automates medical kit production, medical device assembly, medical packaging, and pharmaceutical production for industries such as optical, healthcare, medical devices and diagnostics, healthcare packaging, treatment devices, glucose monitors, syringes, contact lens packaging, MRI and CT scan components, cardiovascular and orthopedic implants, protective equipment, inhalers, safety syringes, pen and auto-injectors, e-devices, IV sets, lancets, pumps and many more. cGMP/GAMP standards are in place for all equipment processes, production, and servicing. RDS combines the knowledge of custom machine building, modular design and state-of-the-art robotics and offers their clients processes that include regulatory guidelines such as UL, VDE, CE, FDA, USDA, GMP and OSHA.
Our systems can process high product volumes using RDS tooling that can also be flexible to change over to different product sizes with quick tool-change capabilities. It is flexible and versatile to comply with evolving industry standards and regulations.
Medical Devices/Products Experience